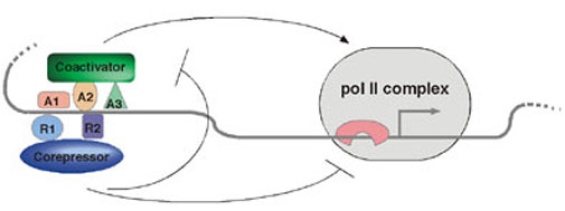
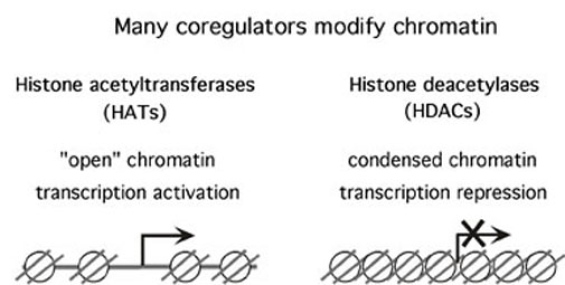
In this project we identify and characterize transcriptional coregulators. Coregulators are proteins that themselves do not bind to DNA, but that facilitate communication between sequence-specific transcription factors and the basal RNA polymerase machinery. One function of coregulators is to modify the structure of chromatin, by acetylating (coactivators) or deacetylating (corepressors) histones.
A number of histone acetyltransferases (HATs) and deacetylases (HDACs) are conserved between organisms as diverse as yeast and human. We are characterizing the role of histone modifying proteins in vivo, using the Drosophila embryo as our “test tube”. We have also isolated novel gene regulators from a genetic screen for mutants that disrupt embryonic pattern formation. Using genetic tools, transgenic embryos, and whole-mount in situ hybridizations in combination with in vitro experiments, we can obtain an understanding of the molecular functions of these proteins in development. Given that coregulators are deregulated in some cancers, these studies will also contribute to an understanding of how altered functions of coregulators can cause uncontrolled cell proliferation and cancer.
An Ebi-HDAC3 corepressor complex
The Snail repressor protein is required for mesoderm formation through its downregulation of neuroectoderm-specific genes. We found that in Drosophila embryos lacking the maternal contribution of the Ebi protein, Snail target genes are de-repressed leading to mis-expression of neuroectoderm specific genes in the presumptive mesoderm. The Ebi protein is the homolog of mammalian TBL1 that associates with HDAC3 and the co-repressors SMRT and NCoR. We have shown that the first 40 amino acids in Snail bind to Ebi in vitro. Interestingly, the first 40 amino acids contain a motif conserved in all insect Snail-related proteins. Importantly, the Ebi interaction region constitutes a potent repression domain. When fused to a heterologous DNA-binding domain, Snail 1-40 represses transcription in Drosophila tissue-culture cells and in transgenic Drosophila embryos. Ectopic expression of full-length Snail and a mutant Snail lacking the Ebi interaction domain in transgenic embryos demonstrated that the Ebi interaction domain is essential for Snail function in vivo. Together, these results show that Ebi is a corepressor required for Snail activity.
We found that Ebi associates with HDAC3 in tissue-culture cells, and that knock down of HDAC3 by RNAi or inhibition of HDAC activity by drug treatment impairs Snail-meditated repression. This shows that histone deacetylation is part of the mechanism by which Snail represses transcription (Qi et al.2008).
The CBP coactivator
One coactivator under study is the CBP protein that is a HAT. We have shown that CBP controls signaling by the TGF-ß molecule Dpp in the Drosophila embryo by multiple mechanisms (Lilja et al. 2003). CBP is necessary for the expression of extracellular regulators of the Dpp protein, as well as for gene activation by Smad proteins that transduce the Dpp signal in the receiving cell. An in vivo structure-function analysis of CBP showed that site-specifically altered CBP cDNA transgenes could rescue the gene expression defects observed in CBP mutant early embryos (Lilja et al. 2007). We found that the acetyltransferase activity of CBP is necessary for some processes during development, but is dispensable for expression of genes in the Dpp pathway in early embryos. Several other co-activators and co-repressors (including histone acetylases and deacetylases) are also under investigation.
References
Qi D. M. Bergman, H. Aihara, Y. Nibu and M. Mannervik. Drosophila Ebi mediates Snail-dependent transcriptional repression through HDAC3-induced histone deacetylation. EMBO J (2008). 27, 898-909.
Lilja, T., D. Qi, M. Stabell, and M. Mannervik, The CBP coactivator functions both upstream and downstream of Dpp/Screw signaling in the early Drosophila embryo. Dev Biol. 2003. 262: 294-302.
Lilja T., H. Aihara, M. Stabell, Y. Nibu and M. Mannervik. The acetyltransferase activity of Drosophila CBP is dispensable for regulation of the Dpp pathway in the early embryo. Dev. Biol. 2007. May 15;305(2):650-8




