Einar HallbergProfessor of Biochemistry
Research
Einar Hallberg, Professor
Signaling across the nuclear envelope
Research Projects
1. The nuclear envelope in cell surface to nucleus signaling
2. Imaging and analysis of cell signaling in live neuronal cells
3. Nuclear envelope proteins in mitosis and maintenance of genomic stability
The nuclear envelope acts as a signaling node to provide enhanced diversification in cell surface to nucleus signaling in eukaryotic cells, a phenomenon which may be especially important in the highly polarized and elongated neuronal cell.
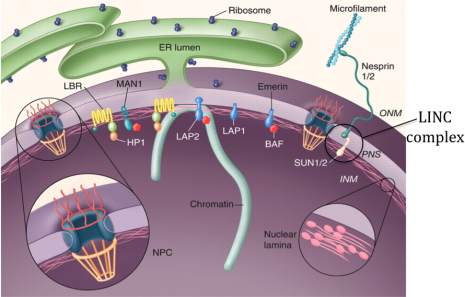
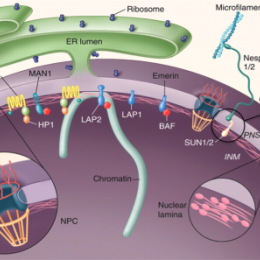
Fig.1. Organization of the nuclear envelope (modified from Stewart et al., 2007)
Background
The nuclear envelope (NE) that surrounds the genetic material in eukaryotic cells (Fig.1) consists of two concentric membranes, the inner nuclear membrane (INM) and outer nuclear membranes (ONM), the nuclear lamina, the nuclear pores and nuclear pore complexes (NPCs). The NPCs are responsible for import and export of proteins and RNA molecules in and out of the nucleus. In addition, a whole new concept in cell signaling bypassing the nuclear pores is provided by the recently discovered LINC complexes (Fig.1), which are built up by specific proteins in the inner and outer nuclear membranes. These direct links across the NE that connect the cytoskeleton with the nuclear interior are believed to mediate mechanical signals from the cell surface to the nuclear interior. The LINC complexes also play important roles in migration of nuclei taking place, e.g. when neuronal cells divide in the developing mammalian brain as well as in muscles for specific positioning of nuclei at neuromuscular junctions.
Human diseases such as cancer have been tied to proteins of the NPC. Nuclear lamins and proteins of the INM have been genetically linked to a diverse group of diseases collectively termed “laminopathies”, which include muscular and lipid dystrophies, neurological disorders and progeria (premature aging). As a result of these discoveries it has now been realized that proteins of the NE orchestrate a much larger repertoire of functions than previously realized, both in cell signaling, chromatin organization and in the mitotic machinery. The INM has been estimated to contain nearly a hundred unique transmembrane proteins, of which the large majority is still not characterized.
Project 1. The nuclear envelope in cell surface to nucleus signaling
Signals from the cell surface may reach the nuclear interior via import of signaling proteins through the nuclear pores (Arabi et al., 2003; Hallberg et al., 1993; Onischenko et al., 2005). However, a whole new concept is provided by the recently discovered LINC complexes, which can mediate direct mechanical transduction of signals from the cytoskeleton to the nuclear interior bypassing the nuclear pores. On the nuclear side the LINC complex interacts with transmembrane proteins of the INM and the nuclear lamina, which may in turn respond by directly or indirectly change chromatin organization and gene activity and or sequester transcription factors.
We are focusing on investigating the function(s) of specific networks of interactions between proteins in the NE and their role in cellular signaling and chromatin organization. As an example, we have recently identified and characterized a novel transmembrane protein from the INM, Samp1 (Buch et al., 2009). Samp1 is conserved from fission yeast to man and contains four conserved Cys-X-X-Cys motifs suggesting that it is able to form two Zinc fingers. Furthermore, Samp1 is essential for connecting the centrosome close to the NE (Buch et al., 2009) and our more recent data strongly suggest that it is connected to the LINC complex (Gudise et al., 2011) and thus may play an important role in cell surface to nucleus signaling.
Project 2. Imaging and analysis of cell signaling in live neuronal cells
We have extensive expertise in imaging and analysis in live cells, including FRAP, FLIP and FRET (Arabi et al., 2003; Buch et al., 2009; Daigle et al., 2001; Eriksson et al., 2004; Figueroa et al., 2011; Ivanova et al., 2016; Le Rouzic et al., 2002; Onischenko et al., 2005; Östlund et al., 1999). The extended tubular shape of neurites addresses particular questions regarding diffusion-mediated signaling in neuronal cells, which may to a large extent rely on motor driven propagation of signals between the cell surface and the nucleus. For example, we have designed a series of anchored FRET (Fluorescences Resonance Energy Transfer) sensor molecules that enable monitoring of local activation of different caspases in neurites and soma of degenerating neuronal cells (Figueroa et al., 2011; Ivanova et al., 2016). We will now use cells expressing these FRET sensors as a model to study mechanisms behind neurodegenerative diseases such as Alzheimers disease (AD). One of our future aims is to develop techniques to image and analyze motor driven signaling in live neuronal cells
Project 3. Nuclear envelope proteins in mitosis and maintenance of genomic stability
Normally, an intricate system of spindle assembly factors and checkpoints assuring correct assembly and maturation of the mitotic spindle is essential for symmetric chromosome segregation and thus maintenance of genomic stability. Defects in the mitotic performance are the main cause of aneuploidy and cancer. Neuroblastoma is the third commonest form of cancer in children and is believed to arise from neuronal progenitor cells during development of the sympathetic nervous system. Genetic instability, manifested as amplification of the MYCN gene, is associated with the aggressive form of the disease.
We use differentiating human neuronal progenitor stem cells as a model system for the development of neuroblastoma in human children. We are focusing on a transmembrane protein of the inner nuclear membrane, Samp1 (Spindle associated membrane proten 1), because it is implicated to play a role in the mitotic machinery and because it specifically concentrates along kinetochore microtubule in mitosis (Fig. 2) (Buch et al., 2009). Recent results show that Samp1 is essential for correct chromosome segregation and binds directly to RanGTPase (Vijayaraghavan et al., 2016), which is a key regulator of mitotic spindle assembly.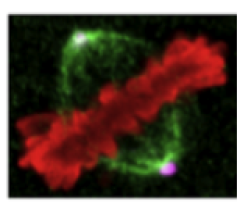
Fig. 2. Samp1, an integral membrane protein of the INM in mitotic spindle membranes. Samp1 (green) concentrates in membranes along kinetochore microtubules of HeLa cells projecting from spindle poles (magenta) towards metaphase chromosomes (red). From (Buch et al., 2009)
Postdoc
Saira Aftab
saira.aftab@dbb.su.se
PhD students
Mehedi Hasan
mehedi.hasan@dbb.su.se
Urska Kasnik
urska.kasnik@dbb.su.se
Veronica Larsson
veronica.larsson@dbb.su.se
Selected publications
Hallberg, E., R.W. Wozniak, and G. Blobel. (1993) An Integral Membrane Protein of the Pore Membrane Domain of the Nuclear Envelope Contains a Nucleoporin-Like Region. J. Cell Biol. 122:513-521.
Östlund, C., J. Ellenberg, E. Hallberg, J. Lippincott-Schwartz, and H.J. Worman. (1999) Intracellular trafficking of emerin, the Emery-Dreifuss muscular dystrophy protein. J. Cell Sci. 112:1709-1719.
Daigle, N., J. Beaudouin, L. Hartnell, G. Imreh, E. Hallberg, J. Lippincott-Schwartz, and J. Ellenberg. (2001) Nuclear pore complexes form immobile networks and have a very low turnover in live mammalian cells. J Cell Biol. 154:71.
Buch, C., R. Lindberg, R. Figueroa, S. Gudise, E. Onischenko, and E. Hallberg. (2009) An integral protein of the inner nuclear membrane localizes to the mitotic spindle in mammalian cells. J Cell Sci. 122:2100.
Figueroa, R.A., V. Ramberg, T. Gatsinzi, M. Samuelsson, M. Zhang, K. Iverfeldt, and E. Hallberg. (2011) Anchored FRET sensors detect local caspase activation prior to neuronal degeneration. Molecular neurodegeneration. 6:35.
Gudise, S., R.A. Figueroa, R. Lindberg, V. Larsson, and E. Hallberg. (2011) Samp1 is functionally associated with the LINC complex and A-type lamina networks. J Cell Sci. 124:2077-2085.
Bergqvist, C., Jafferali, M.H., Gudise, S., Markus, R., Bergqvist, C. and Hallberg, E. (2017) An inner nuclear membrane protein induces rapid differentiation of human induced pluripotent stem cells. Stem Cell Res., 23:33-38.
Jafferali, M.H., Figueroa, R.A., Hasan, M. and Hallberg, E. (2017) Spindle associated membrane protein 1 (Samp1) is required for the differentiation of muscle cells. Sci. Rep. 7: 16655.
Larsson, V., Figueroa, R., Jafferali, M., Vijayaraghavan, B., and Hallberg, E. (2018) Mitotic spindle assembly and g-tubulin localisation depend on the integral nuclear membrane protein, Samp1. J. Cell Sci. 131, jcs211664.
Bergqvist, C., Niss, F., Figueroa, R., Beckman, M., Maksel, D., Jafferali, M.H., Kulyté, A., Ström, A. and Hallberg, E. (2019) Monitoring of chromatin organization in live cells by FRIC. Effects of the inner nuclear membrane protein Samp1. Nucleic Acids Research, 2019, Vol. 47 (9) e49 doi: 10.1093/nar/gkz123.
Research projects
Publications
A selection from Stockholm University publication database
-
Monitoring of chromatin organization in live cells by FRIC. Effects of the inner nuclear membrane protein Samp1
2019. Cecilia Bergqvist (et al.). Nucleic Acids Research 47 (9)
ArticleRead more about Monitoring of chromatin organization in live cells by FRIC. Effects of the inner nuclear membrane protein Samp1In most cells, transcriptionally inactive heterochromatin is preferentially localized in the nuclear periphery and transcriptionally active euchromatin is localized in the nuclear interior. Different cell types display characteristic chromatin distribution patterns, which change dramatically during cell differentiation, proliferation, senescence and different pathological conditions. Chromatin organization has been extensively studied on a cell population level, but there is a need to understand dynamic reorganization of chromatin at the single cell level, especially in live cells. We have developed a novel image analysis tool that we term Fluorescence Ratiometric Imaging of Chromatin (FRIC) to quantitatively monitor dynamic spatiotemporal distribution of euchromatin and total chromatin in live cells. A vector (pTandemH) assures stoichiometrically constant expression of the histone variants Histone 3.3 and Histone 2B, fused to EGFP and mCherry, respectively. Quantitative ratiometric (H3.3/H2B) imaging displayed a concentrated distribution of heterochromatin in the periphery of U2OS cell nuclei. As proof of concept, peripheral heterochromatin responded to experimental manipulation of histone acetylation. We also found that peripheral heterochromatin depended on the levels of the inner nuclear membrane protein Samp1, suggesting an important role in promoting peripheral heterochromatin. Taken together, FRIC is a powerful and robust new tool to study dynamic chromatin redistribution in live cells.
-
Mitotic spindle assembly and γ-tubulin localisation depend on the integral nuclear membrane protein, Samp1
2018. Veronica J. Larsson (et al.). Journal of Cell Science
ArticleRead more about Mitotic spindle assembly and γ-tubulin localisation depend on the integral nuclear membrane protein, Samp1We have investigated a possible role of the inner nuclear membrane protein, Samp1, in the mitotic machinery. Live cell imaging showed that Samp1aYFP distributed as filamentous structures in the mitotic spindle, partially co-localising with ß-tubulin. Samp1 depletion resulted in an increased frequency of cells with signs of chromosomal mis-segregation and prolonged metaphase, indicating problems with spindle assembly and/or chromosomal alignment. Consistently, mitotic spindles in Samp1 depleted cells contained significantly lower levels of ß-tubulin and γ-tubulin, phenotypes which were rescued by overexpression of Samp1aYFP. We found that Samp1 can bind directly to γ-tubulin and that Samp1 co-precipitated with γ-tubulin and HAUS6 of the Augmin complex in live cells. The levels of Haus6, in the mitotic spindle also decreased after Samp1 depletion. We show that Samp1 is involved in the recruitment of Haus6 and γ-tubulin to the mitotic spindle. Samp1 is the first inner nuclear membrane protein shown to have a function in mitotic spindle assembly.
-
Read more about RanGTPase regulates the interaction between the inner nuclear membrane proteins, Samp1 and Emerin
RanGTPase regulates the interaction between the inner nuclear membrane proteins, Samp1 and Emerin
Balaje Vijayaraghavan (et al.).
-
An inner nuclear membrane protein induces rapid differentiation of human induced pluripotent stem cells
2017. Cecilia Bergqvist (et al.). Stem Cell Research 23, 33-38
ArticleRead more about An inner nuclear membrane protein induces rapid differentiation of human induced pluripotent stem cellsThe ability of iPSCs (induced pluripotent stem cells) to generate any cell type in the body makes them valuable tools for cell replacement therapies. However, differentiation of iPSCs can be demanding, slowand variable. During differentiation chromatin is re-organized and silent dense heterochromatin becomes tethered to the nuclear periphery by processes involving the nuclear lamina and proteins of the INM(inner nuclearmembrane). The INM protein, Samp1 (Spindle AssociatedMembrane Protein 1) interacts with Lamin A/C and the INMprotein Emerin, which has a chromatin binding LEM(Lap2-Emerin-Man1)-domain. In this paperweinvestigate if Samp1 can play a role in the differentiation of iPSCs. Samp1 levels increased as differentiating iPSCs started to express Lamin A/C. Interestingly, even under pluripotent culturing conditions, ectopic expression of Samp1 induced a rapid differentiation of iPSCs, ofwhich some expressed the neuronal marker beta III-tubulin already after 6 days. This suggests that Samp1 is involved in early differentiation of iPSCs and could potentially be explored as a tool to promote progression of the differentiation process.
-
Molecular basis for the dual subcellular distribution of microsomal glutathione transferase 1
2017. Miyuki Shimoji (et al.). Biochimica et Biophysica Acta - Biomembranes 1859 (2), 238-244
ArticleRead more about Molecular basis for the dual subcellular distribution of microsomal glutathione transferase 1Microsomal glutathione transferase 1 (MGST1) is a membrane bound enzyme involved in the detoxification of reactive electrophiles and protection of membranes from oxidative stress. The enzyme displays an unusual and broad subcellular distribution with especially high levels in the endoplasmic reticulum (ER) and outer mitochondrial membrane (OMM). Here we examined the molecular basis for this dual distribution. We hypothesized that the amphipathic properties of the first transmembrane segment (TMS), that contains a positively charged lysine (K25), is a central feature guiding dual targeting. The lysine-25 was substituted to alanine by site directed mutagenesis. We also increased the amphipathic character of the helix by inserting an additional lysine either one turn above or below K25. Expressing these constructs in simian COS cells, and analyzing subcellular distribution by immunocytochemistry, we observed an increased ER targeting of K25A-MGST1. In contrast I22K-MGST1 and F28K-MGST1 displayed pronounced mitochondrial targeting. By using in vitro transcription-translation we examined whether insertion of WT-MGST1 into ER is co- or post-translational and provide evidence for the former. In the same experimental set-up, mitochondrial insertion was shown to depend on the positive charge. Together these results show that removing the positive charge of lysine-25 promotes ER incorporation, but counteracts mitochondrial insertion. In contrast, introducing an extra lysine in the first TMS of MGST1 had opposite effects. The amphipathic character of the first TMS thus constitutes a molecular determinant for the dual targeting of MGST1. Broad subcellular distribution is consistent with a physiological role in protection from reactive intermediates and oxidative stress.
-
Nuclear envelope localization of LEMD2 is developmentally dynamic and lamin A/C dependent yet insufficient for heterochromatin tethering
2017. Katharina Thanisch (et al.). Differentiation 94, 58-70
ArticleRead more about Nuclear envelope localization of LEMD2 is developmentally dynamic and lamin A/C dependent yet insufficient for heterochromatin tetheringPeripheral heterochromatin in mammalian nuclei is tethered to the nuclear envelope by at least two mechanisms here referred to as the A- and B-tethers. The A-tether includes lamins A/C and additional unknown components presumably INM protein(s) interacting with both lamins A/C and chromatin. The B tether includes the inner nuclear membrane (INM) protein Laurin B-receptor, which binds B-type lamins and chromatin. Generally, at least one of the tethers is always present in the nuclear envelope of mammalian cells. Deletion of both causes the loss of peripheral heterochromatin and consequently inversion of the entire nuclear architecture, with this occurring naturally in rod photoreceptors of nocturnal mammals. The tethers are differentially utilized during development, regulate gene expression in opposite manners, and play an important role during cell differentiation. Here we aimed to identify the unknown chromatin binding component(s) of the A-tether. We analyzed 10 mouse tissues by immunostaining with antibodies against 7 INM proteins and found that every cell type has specific, although differentially and developmentally regulated, sets of these proteins. In particular, we found that INM protein LEMD2 is concomitantly expressed with A-type lamins in various cell types but is lacking in inverted nuclei of rod cells. Truncation or deletion of Lmna resulted in the downregulation and mislocalization of LEMD2, suggesting that the two proteins interact and pointing at LEMD2 as a potential chromatin binding mediator of the A-tether. Using nuclei of mouse rods as an experimental model lacking peripheral heterochromatin, we expressed a LEMD2 transgene alone or in combination with lamin C in these cells and observed no restoration of peripheral heterochromatin in either case. We conclude that in contrary to the B-tether, the A-tether has a more intricate composition and consists of multiple components that presumably vary, at differing degrees of redundancy, between cell types and differentiation stages.
-
Role of autophagy in cell-penetrating peptide transfection model
2017. Moataz Dowaidar (et al.). Scientific Reports 7
ArticleRead more about Role of autophagy in cell-penetrating peptide transfection modelCell-penetrating peptides (CPPs) uptake mechanism is still in need of more clarification to have a better understanding of their action in the mediation of oligonucleotide transfection. In this study, the effect on early events (1 h treatment) in transfection by PepFect14 (PF14), with or without oligonucleotide cargo on gene expression, in HeLa cells, have been investigated. The RNA expression profile was characterized by RNA sequencing and confirmed by qPCR analysis. The gene regulations were then related to the biological processes by the study of signaling pathways that showed the induction of autophagy-related genes in early transfection. A ligand library interfering with the detected intracellular pathways showed concentration-dependent effects on the transfection efficiency of splice correction oligonucleotide complexed with PepFect14, proving that the autophagy process is induced upon the uptake of complexes. Finally, the autophagy induction and colocalization with autophagosomes have been confirmed by confocal microscopy and transmission electron microscopy. We conclude that autophagy, an inherent cellular response process, is triggered by the cellular uptake of CPP-based transfection system. This finding opens novel possibilities to use autophagy modifiers in future gene therapy.
-
Anchoring of FRET Sensors-A Requirement for Spatiotemporal Resolution
2016. Elena V. Ivanova (et al.). Sensors 16 (5)
ArticleRead more about Anchoring of FRET Sensors-A Requirement for Spatiotemporal ResolutionFRET biosensors have become a routine tool for investigating mechanisms and components of cell signaling. Strategies for improving them for particular applications are continuously sought. One important aspect to consider when designing FRET probes is the dynamic distribution and propagation of signals within living cells. We have addressed this issue by directly comparing an anchored (taFS) to a non-anchored (naFS) cleavable FRET sensor. We chose a microtubule-associated protein tau as an anchor, as microtubules are abundant throughout the cytosol of cells. We show that tau-anchored FRET sensors are concentrated at the cytoskeleton and enriched in the neurite-like processes of cells, providing high intensity of the total signal. In addition, anchoring limits the diffusion of the sensor, enabling spatiotemporally resolved monitoring of subcellular variations in enzyme activity. Thus, anchoring is an important aspect to consider when designing FRET sensors for deeper understanding of cell signaling.
-
MCLIP Detection of Novel Protein-Protein Interactions at the Nuclear Envelope
2016. Mohammed Hakim Jafferali, Ricardo A. Figueroa, Einar Hallberg. Intermediate Filament Associated Proteins, 503-515
ChapterRead more about MCLIP Detection of Novel Protein-Protein Interactions at the Nuclear EnvelopeThe organization and function of the nuclear envelope (NE) involves hundreds of nuclear membrane proteins and myriad protein-protein interactions, most of which are still uncharacterized. Many NE proteins interact stably or dynamically with the nuclear lamina or chromosomes. This can make them difficult to extract under non-denaturing conditions, and greatly limits our ability to explore and identify functional protein interactions at the NE. This knowledge is needed to understand nuclear envelope structure and the mechanisms of human laminopathy diseases. This chapter provides detailed protocols for MCLIP (membrane cross-linking immunoprecipitation) identification of novel protein-protein interactions in mammalian cells.
-
Samp1, a RanGTP binding transmembrane protein in the inner nuclear membrane
2016. Balaje Vijayaraghavan (et al.). Nucleus 7 (4), 415-423
ArticleRead more about Samp1, a RanGTP binding transmembrane protein in the inner nuclear membraneSamp1 is a transmembrane protein of the inner nuclear membrane (INM), which interacts with the nuclear lamina and the Linker of Nucleoskeleton and Cytoskeleton (LINC) complex in interphase and during mitosis, it localizes to the mitotic spindle. Samp1 was recently found to coprecipitate a protein complex containing Ran, a GTPase with fundamental regulatory functions both in interphase and in mitosis. To investigate the interaction between Samp1 and Ran in further detail, we have designed and expressed recombinant fusion proteins of the Chaetomium thermophilum homolog of Samp1 (Ct. Samp1) and human Ran. Pulldown experiments show that Samp1 binds directly to Ran and that Samp1 binds better to RanGTP compared to RanGDP. Samp1 also preferred RanGTP over RanGDP in living tsBN2 cells. We also show that the Ran binding domain is located between amino acids 75-135 in the nucleoplasmically exposed N-terminal tail of Samp1. This domain is unique for Samp1, without homology in any other proteins in fungi or metazoa. Samp1 is the first known transmembrane protein that binds to Ran and could provide a unique local binding site for RanGTP in the INM. Samp1 overexpression resulted in increased Ran concentrations in the nuclear periphery supporting this idea.
-
A convergent uptake route for peptide- and polymer-based nucleotide delivery systems
2015. Staffan Lindberg (et al.). Journal of Controlled Release 206, 58-66
ArticleRead more about A convergent uptake route for peptide- and polymer-based nucleotide delivery systemsCell-penetrating peptides (CPPs) have been used as vehicles to deliver various cargos into cells and are promising as tools to deliver therapeutic biomolecules such as oligonucleotides both in vitro and in vivo. CPPs are positively charged and it is believed that CPPs deliver their cargo in a receptor-independent manner by interactingwith the negatively charged plasmamembrane and thereby inducing endocytosis. In this study we examine the mechanism of uptake of several different, well known, CPPs that form complexes with oligonucleotides.We show that these CPP:oligonucleotide complexes are negatively charged in transfection-media and their uptake is mediated by class A scavenger receptors (SCARA). These receptors are known to promiscuously bind to, and mediate uptake of poly-anionic macromolecules. Uptake of CPP:oligonucleotide complexes was abolished using pharmacological SCARA inhibitors as well as siRNA-mediated knockdown of SCARA. Additionally, uptake of CPP:oligonucleotide was significantly increased by transiently overexpressing SCARA. Furthermore, SCARA inhibitors also blocked internalization of cationic polymer:oligonucleotide complexes.Our results demonstrate that the previous held belief that CPPs act receptor independently does not hold true for CPP:oligonucleotide complexes, as scavenger receptor class A (SCARA) mediates the uptake of all the examined CPP:oligonucleotide complexes in this study.
-
MCLIP, an effective method to detect interactions of transmembrane proteins of the nuclear envelope in live cells
2014. Mohammed Hakim Jafferali (et al.). Biochimica et Biophysica Acta - Biomembranes 1838 (10), 2399-2403
ArticleRead more about MCLIP, an effective method to detect interactions of transmembrane proteins of the nuclear envelope in live cellsInvestigating interactions of proteins in the nuclear envelope (NE) using co-immunoprecipitation (Co-IP) has previously been difficult or even impossible due to their inherent resistance to extraction. We have developed a novel method, MCLIP (Membrane protein Cross-Link ImmunoPrecipitation), which takes advantage of a cell permeable crosslinker to enable effective detection and analysis of specific interactions of NE proteins in live cells using Western blot. Using MCLIP we show that, in U2OS cells, the integral inner nuclear membrane protein Samp1 interacts with Lamin B1, the LINC (Linker of nucleoskeleton and cytoskeleton) complex protein, Sun1 and the soluble small GTPase Ran. The results show that the previously detected in vitro interaction between Samp1 and Emerin also takes place in live cells. In vitro pull down experiments show, that the nucleoplasmic domains of Samp1 and Emerin can bind directly to each other. We also, show that MCLIP is suitable to coprecipitate protein interactions in different stages of the cell cycle.
-
Anchored FRET sensors detect local caspase activation prior to neuronal degeneration
2011. Ricardo A. Figueroa (et al.). Molecular Neurodegeneration 6, 35
ArticleRead more about Anchored FRET sensors detect local caspase activation prior to neuronal degenerationBACKGROUND: Recent studies indicate local caspase activation in dendrites or axons during development and in neurodegenerative disorders such as Alzheimer's disease (AD). Emerging evidences point to soluble oligomeric amyloid-beta peptide as a causative agent in AD.
RESULTS: Here we describe the design of fluorescence resonance energy transfer (FRET)-based caspase sensors, fused to the microtubule associated protein tau. Specific caspase sensors preferentially cleaved by caspase-3, -6 or -9 were expressed in differentiated human neuroblastoma SH-SY5Y cells. The anchoring of the sensors resulted in high FRET signals both in extended neurites and soma and made analysis of spatiotemporal signal propagation possible. Caspase activation was detected as loss of FRET after exposure to different stimuli. Interestingly, after staurosporine treatment caspase-6 activation was significantly delayed in neurites compared to cell bodies. In addition, we show that exposure to oligomer-enriched amyloid-beta peptide resulted in loss of FRET in cells expressing sensors for caspase-3 and -6, but not -9, in both soma and neurites before neurite degeneration was observed.
CONCLUSIONS: Taken together, the results show that by using anchored FRET sensors it is possible to detect stimuli-dependent differential activation of caspases and to distinguish local from global caspase activation in live neuronal cells. Furthermore, in these cells oligomer-enriched amyloid-beta peptide induces a global, rather than local activation of caspase-3 and -6, which subsequently leads to neuronal cell death.
-
Microtubule-associated nuclear envelope proteins in interphase and mitosis
2011. Ricardo A. Figueroa, Santhosh Gudise, Einar Hallberg. Biochemical Society Transactions 39, 1786-1789
ArticleRead more about Microtubule-associated nuclear envelope proteins in interphase and mitosisThe LINC (linker of nucleoskeleton and cytoskeleton) complex forms a transcisternal bridge across the NE (nuclear envelope) that connects the cytoskeleton with the nuclear interior. This enables some proteins of the NE to communicate with the centrosome and the microtubule cytoskeleton. The position of the centrosome relative to the NE is of vital importance for many cell functions, such as cell migration and division, and centrosomal dislocation is a frequent phenotype in laminopathic disorders. Also in mitosis, a small group of transmembrane NE proteins associate with microtubules when they concentrate in a specific membrane domain associated with the mitotic spindle. The present review discusses structural and functional aspects of microtubule association with NE proteins and how this association may be maintained over the cell cycle.
-
Samp1 is functionally associated with the LINC complex and A-type lamina networks
2011. Santhosh Gudise (et al.). Journal of Cell Science 124, 2077-2085
ArticleRead more about Samp1 is functionally associated with the LINC complex and A-type lamina networksThe transmembrane inner nuclear membrane (INM) protein Samp1 is required for anchoring centrosomes near the nuclei. Using high-resolution fluorescence microscopy we show that Samp1 is distributed in a distinct and characteristic pattern in the nuclear envelope (NE), where it partially colocalizes with the LINC complex protein Sun1. By studying the localization of Samp1 deletion mutants and fusion proteins, we conclude that the cysteine-rich N-terminal half of Samp1 is nucleoplasmically exposed and is responsible for targeting to the INM. It contains four conserved CxxC motifs with the potential to form zinc fingers. The distribution of cysteine-to-alanine substitution mutants, designed to prevent zinc finger formation, showed that NE localization of Samp1 depends on intact CxxC motifs. Overexpression of Samp1 zinc finger mutants produced an abnormal dominant phenotype characterized by disrupted organization of a selective subset NE proteins, including emerin, Sun1, endogenous Samp1 and, in some cases, lamin A/C, but not lamin B, Sun2 or nucleoporins. Silencing of Samp1 expression showed that emerin depends on Samp1 for its correct localization in the NE. Our results demonstrate that Samp1 is functionally associated with the LINC complex protein Sun1 and proteins of the A-type lamina network.
-
A transmembrane inner nuclear membrane protein in the mitotic spindle
2010. Ricardo Figueroa (et al.). Nucleus (Austin) 1 (3), 249-253
ArticleRead more about A transmembrane inner nuclear membrane protein in the mitotic spindleWe have recently characterized a novel transmembrane protein of the inner nuclear membrane of mammalian cells. The protein has two very interesting features. First, despite being an integral membrane protein it is able to concentrate in the membranes colocalizing with the mitotic spindle in metaphase and anaphase. Hence, the protein was named Samp1, Spindle associated membrane protein 1. Secondly, it displays a functional connection to centrosomes. This article discusses various aspects of Samp1 in relation to possible cellular function(s).
-
Nucleus and Nuclear Envelope
2010. Marie Beckman, Madeleine Kihlmark, Einar Hallberg.
BookRead more about Nucleus and Nuclear EnvelopeThe cell nucleus of eukaryotic organisms contains the genome surrounded by a nuclear envelope consisting of a double-lipid membrane with embedded nuclear pores and an underlying nuclear lamina. The uniformity in size and density makes it possible to isolate pure intact nuclei at high yields from tissue homogenates by centrifugation through a sucrose cushion. Nuclear envelopes can be prepared from isolated nuclei by enzymatic degradation of their nucleic acid content. The resulting nuclear envelope preparations contain structurally well-conserved inner and outer nuclear membranes with attached ribosomes, nuclear pore complexes and nuclear lamina. Reliable methods for preparation of nuclei and nuclear envelopes play an important role in the successful identification of components that are located in nuclei and in nuclear subcompartments.
-
Read more about Spindle associated membrane protein 1 (Samp1) is required for the differentiation of muscle cells
Spindle associated membrane protein 1 (Samp1) is required for the differentiation of muscle cells
Mohammed Hakim Jafferali (et al.).
Show all publications by Einar Hallberg at Stockholm University