European chemicals regulation needs greater transparency
May 2019: To meet the challenges of chemical risks in everyday life, risk assessments under the REACH registration process must be improved. One key element in this transformation is to introduce greater transparency.
RECOMMENDATIONS
To the European Commission:
• Investigate and suggest changes in the REACH legislation that further increase its transparency.
• Investigate the possible negative consequences of conflicts of interest in the REACH registration process, and consider the option of having an independent third party perform chemical risk assessments.
• Increase the number of dossiers that ECHA has to review in order to promote increased quality of chemical risk assessments.
To the European Chemicals Agency:
• Further explore how dissemination of risk assessments and toxicity studies can be enhanced within the current REACH legislation.
• Implement and enforce a common method with clear criteria and guidance for evaluating toxicity studies, as well as a template for transparent reporting of assessments.
To the chemical industry:
• Support transparency initiatives and make the toxicity studies used for concluding on chemical risks fully available for independent scrutiny.

In our daily lives we use a wide range of chemical-intensive products such as construction materials, textiles, cars, electronics and toys – and the use of chemicals in society is increasing every year. This requires an improved ability to understand, identify and manage potential chemical risks to human health and the environment.
In 2007, the European chemicals legislation REACH (Registration, Evaluation, Authorisation, and Restriction of Chemicals) was adopted to ensure that risks with chemicals are adequately controlled. However, the processes for identifying and taking appropriate regulatory measures against potentially harmful chemicals are still slow in relation to the rate that chemicals are being put on the European market.
To meet the current challenges regarding chemicals in everyday life, the structures for the control and gathering of chemical information under the legislation must be improved. One action that might improve the preconditions for using REACH to identify chemicals of concern for human health and the environment is to introduce significantly greater transparency in the registration process.
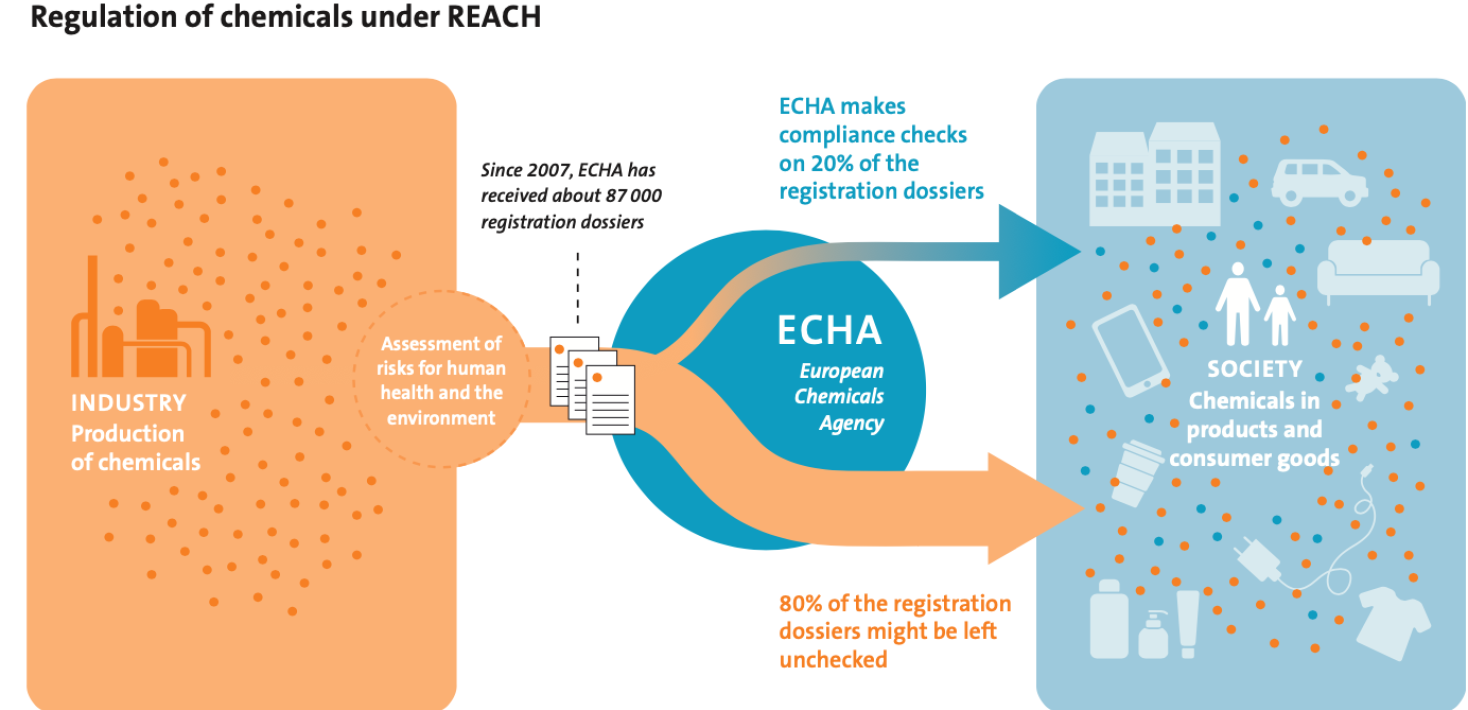
Why is transparency needed?

Identifying and managing chemicals of concern is a cornerstone of the REACH legislation. How well this is done depends heavily on the quality of the risk assessments provided by the chemical industry to ECHA.
These assessments rely on information about the chemical’s toxicity, uses, and foreseen exposures and risks, as well as instructions for safe handling. The current distribution of responsibilities and the characteristics of the REACH legislation create challenges that are directly relevant to transparency:
• First, the registration process has an inherent conflict of in- terest. The responsibility for generating and evaluating (eco) toxicity data and performing the risk assessment lies with the producer or importer of the chemical, i.e. an actor that has a clear economic interest in the outcome of the process.
• Second, it is unclear if the REACH legislation can deliver comprehensive and correct assessments that are suitable for decision-making. Both the German Federal Institute for Risk Assessment (BfR) and the European Commission have performed evaluations of REACH registrations – and they both conclude that there is a remarkable lack of compliance from the chemicals industry and that the information provided is often not sufficient for authorities to identify and prioritise the need for action.
• Third, ECHA performs compliance checks on a relatively small percentage of the registered risk assessments (20% per tonnage band). Consequently, 80% of the risk assessments provided by the industry are not checked for legal compliance.
As of this year, ECHA increased the percentage of compliance checks from 5% to 20% per tonnage band. This is an improvement, but considering the high number of non-compliant dossiers this target has to increase further until compliance is significantly improved. Furthermore, the increased compliance check target should be combined with a demand for greater scope in each control because current compliance checks only address certain parts of the scrutinised dossiers.
To ensure protection of human health and the environment, there is an urgent need for increased supervision and evaluation of the quality of the risk assessments performed under REACH. This calls for complete transparency so that independent evaluation by third parties is possible.
FIVE REASONS WHY REACH NEEDS GREATER TRANSPARENCY
European chemical safety is built on a regulatory system that:
• apparently provides insufficient guidance to registrants on how to evaluate and summarise toxicity studies
• is susceptible to bias because it has an inherent conflict of interest relying on industry to show that the risks with their products are adequately controlled
• repeatedly has been shown to have a remarkably high level of non-compliance
• provides responsible agencies with limited resources to ensure compliance
• offers limited possibilities for third parties to scrutinise the risk assessments made by industry
Source: Transparency within REACH? Regulatory risk assessment of industrial chemicals (Stockholm University, 2018).
Poor reporting of environmental and health information

According to REACH, producers or importers of chemicals must report all available and relevant (eco)toxicological information in sufficient detail needed to fully understand the reasoning and conclusion of their risk assessment. The information is collected in a dossier and provided to ECHA.
Unfortunately, the quality of these dossiers varies greatly. As a consequence, it is not always possible to understand and thereby assess how the producers or importers arrive at their conclusions on risk.
In a recent thesis from Stockholm University, Transparency within REACH? Regulatory risk assessment of industrial chemicals (2018), scientists examined 60 REACH registration dossiers, focusing on how scientific information was reported and used. The results show considerable variation in the quality of data evaluation and reporting. Among the observed discrepancies were omitted information on the design of toxicological studies, and incomplete reporting of toxicological effects. In addition, the amount of information registered varied greatly, from reporting comprehensive environmental and health information to merely providing a few summarising sentences.
ECHA’s current guidance on how to report scientific information for chemical assessments fails to guide the industry in delivering information suitable for decision-making.
Poor possibilities to scrutinise information

Confidential business information and intellectual property rights have strong protection in law. Consequently, toxicity studies commissioned by the chemical industry are not publicly available, and third parties, such as scientists and NGOs, have little or no possibility to use or scrutinise the information behind the risk assessments.
One way of increasing transparency is to make such confidential information public. This would require legislative changes or a different interpretation of the existing law on disclosure of information.
The movement towards increased transparency is already ongoing within the EU. In 2019, the European Parliament and the Council reached an agreement regarding the Commission’s proposal to boost transparency in EU’s General Food Law. The reform will require industry to make publicly available the complete toxicity studies used in risk assessments of chemicals that end up in our food, such as pesticides and food additives. It is equally important to also make a similar reform for the REACH legislation.
Authors and contact
This policy brief is based on the thesis: Transparency within REACH? Regulatory risk assessment of industrial chemicals, by Ellen Ingre-Khans at the Department of Environmental Science and Analytical Chemistry (ACES), Stockholm University.
Policy Brief authors
Christina Rudén and Marlene Ågerstrand, Department of Environmental Science and Analytical Chemistry (ACES), Stockholm University.
For questions or more information, please contact the authors at christina.ruden@aces.su.se or marlene.agerstrand@aces.su.se.
Read this policy brief as a layouted pdf
Last updated: February 24, 2022
Source: Östersjöcentrum

