Research group PGA group
Stereoselective synthesis is a crucial process in organic chemistry and is extremely important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity.
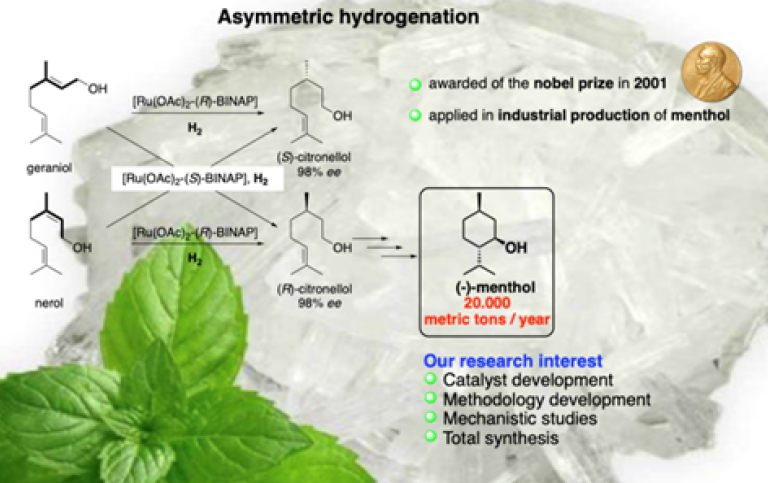
For the past two decades, our group has gained experience in the field of metal-catalyzed asymmetric hydrogenation. During this period, novel metal catalysts were developed and applied in the hydrogenation of various classes of substrates, including challenging aliphatic- and tetrasubstituted olefins. Recent developed protocols include advances in regioselective hydrogenation of dienes, synthesis of chiral fluorine motifs and dynamic kinetic resolution of allylic alcohols.
Current interests lay in:
- Methodology development using transition metal-catalyzed asymmetric hydrogenation, such as;
- Enantioconvergent hydrogenation where both E- and Z-olefins lead to the same absolute configuration of the product.
- (Dynamic) kinetic resolution of various classes of functionalized olefins.
- Synthesis of chiral fluorine scaffolds.
- Application of developed methodology in total synthesis of natural products and pharmaceuticals.
Group members
Group managers
Pher Andersson
Professor
Members
Rajendra Kumar Mallick
Postdok


