Research project Metabolic regulation and spatio-temporal aspects of fungal virulence in situ
Amino acids are essential nutrients that serve as building blocks of proteins and some are efficiently metabolized for energy. Eukaryotic cells respond to extracellular amino acids by enhancing their uptake. We study the molecular mechanisms underlying this response and the role of amino acid metabolism in promoting virulent growth of human fungal pathogens.
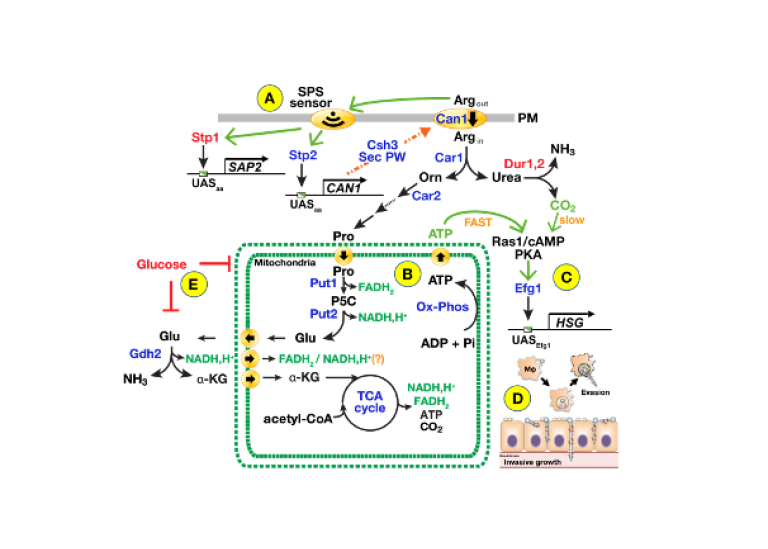
Humans host commensal microorganisms known as the human microflora that is largely composed of bacteria, however, eukaryotic fungi are major components with Candida spp. dominating. Many Candida species are opportunistic pathogens that cause life threating infections in immuno-compromised individuals. The incidence of candidiasis is low in healthy populations, indicating that interactions with immune cells play vital roles. We identified mitochondrial proline catabolism as critical for inducing filamentous growth of C. albicans cells, a virulence feature that enables cells to cross endo- and epithelial barriers and evade from macrophages. Building on this discovery we are: 1) characterizing the mitochondrial-localized processes that are critical to fungal cell survival; 2) visualizing the spatio-temporal aspects of C. albicans infections in the kidney of a living host and defining host-pathogen interactions using intravital 2-photon and STED microscopy and spatio-transcriptomic analysis; and 3) defining the virulence properties of multidrug resistant Candida auris.
Project description
The human microflora is composed of a wide spectrum of commensal microorganisms including fungi, with Candida spp. dominating. Many species of Candida, e.g., Candida albicans, Candida glabrata and Candida auris, are opportunistic pathogens that can cause life threating infections in immune compromised individuals. As the incidence of candidiasis is quite low in healthy populations, environmental factors, such as interactions with the primary immune cells play critical roles. Our studies in the non-pathogenic baker’s yeast Saccharomyces cerevisiae (see project Nutrient Regulated Gene Expression in Eukaryotic Cells) have established paradigms to understand nutrient-regulated processes in Candida spp. However, fundamental and important differences exist. In constrast to yeast, which have evolved in high-glucose environments and are readily be found in nature, Candida spp. have evolved in close association with human hosts and are not found living freely in nature. Candida spp. are well-adapted for growth in the low glucose environment of human hosts and efficently metabolize amino acids as energy sources. There are high levels of amino acids in circulateing blood, and host proteins are rich sources of amino acids that can be liberated by host or fungal proteases that cleave them into smaller peptides and free amino acids. The advent of CRISPR/Cas9-based methods have facilitated the genetic analysis of human fungal pathogens and we are applying state-of-the-art molecular biological techniques to directly examine growth requirements in vivo and in situ in infected model mammalian hosts. We have recently identified mitochondrial proline catabolism as critical for inducing and energizing filamentous growth, a virulence feature that underlies evasion from macrophages and the ability to invade across endo- and epithelial barriers (see Figure 1). Building on this knowledge, we are pursuing three aims: 1) Fully characterize the metabolic control of proline-dependent fungal virulence, specifically the role of mitochondrial-localized processes that are critical to fungal cell survival in hosts; 2) Visualize the spatio-temporal aspects of C. albicans infections in the kidney of a living mammalian host and define host-pathogen interactions using advanced intravital 2-photon and STED microscopy and spatio-transcriptomic analysis; and 3) Define the virulence properties of multidrug resistant Candida auris. We anticipate that the results will provide a solid foundation for developing novel therapeutic strategies in the expanding population of immune compromised individuals.
Project members
Project managers
Per Ljungdahl
Professor