Research project Multitracer imaging for personalized dose prescription: from modelling to clinical implementation
A personalized radiotherapy strategy, making use of the tumour functional information, which aims at administering a more aggressive treatment in the areas of the tumour more resistant to radiation.
According to the Swedish Head and Neck Cancer Register, the incidence of head and neck cancer in Sweden is approximately 1,500 cases per year (+25% with respect to the ten-year period from 2008-2017), which constitutes 2.3% of all cancers. In about two-thirds of the cases, head
and neck cancers are detected at an advanced stage of the disease where the prognosis remains poor with about half of the patients developing tumour recurrences within 2 years from treatment. A multidisciplinary approach, including the use of radiotherapy, is normally used in the advanced stage of the disease. Since tumour recurrence is often found within the main radiotherapy target areas, this indicates that a more aggressive and tailored radiation treatment would be required.
The proposed project is designed around the patient and is conducive to a concrete clinical implementation where the improvement of the patient quality of life and survival are the primary objectives. This project develops following a twofold strategy: I) it aims at administering a more aggressive radiation treatment only in the areas of the tumour which are identified to be more resistant to radiation while sparing the surrounding healthy tissues; and II) it aims at identifying, at an
early time point, the patients that are not responding well to radiotherapy. An early identification of the non-responders will allow for a prompt change in the strategy of treatment while there is still time to transform the fate of the treatment into a successful one. This personalized strategy of radiotherapy makes use of the tumour functional information and, in particular, of the information on metabolism and oxygen content of the tumour. The radiation treatment is envisaged to be administered through a particular type of radiation (protons) that is able to more efficiently spare the healthy tissues while targeting the radiation to the tumour.
Project description
The project will address the following fundamental questions:
-Is it possible to find a robust dose painting strategy based on the hypoxia Positron Emission Tomography (PET) imaging, which can bridge the
resolution gap between the sub-millimetre scale at which hypoxia manifests and the PET resolution scale?
-Is it possible to predict at an early time point what would be the treatment outcome and, thus, identify responders/non-responders to
treatment?
-If an early identification of patients not responding to treatment is possible, how could this information be used and how can the treatment be adapted?
The following research directions are pursued:
Photon and proton therapy outcome assessment with dose painting prescription based on an in silico oxygen distribution model.
(a) In silico modelling of tumour hypoxia will be used to bridge the resolution gap between the microscopic scale at which hypoxia manifests and the PET imaging scale. The influence of the PET imaging resolution in the assessment of different hypoxia levels and in the prescription of
corresponding radiotherapeutic doses will be quantified.
(b) Different dose painting strategies based on hypoxia PET imaging will be consider and the optimal “brush size” for dose painting determined.
Clinical feasibility in delivering the considered dose painting strategies will be determined in terms of target coverage and organ at risk constraints for different beam qualities (i.e. photons and protons). An assessment of the treatment outcome prediction in terms of Tumour
Control Probability (TCP) accounting for the underlying oxygen distribution at sub-millimetre scale as well as normal tissue complication probability (NTCP) will be performed.
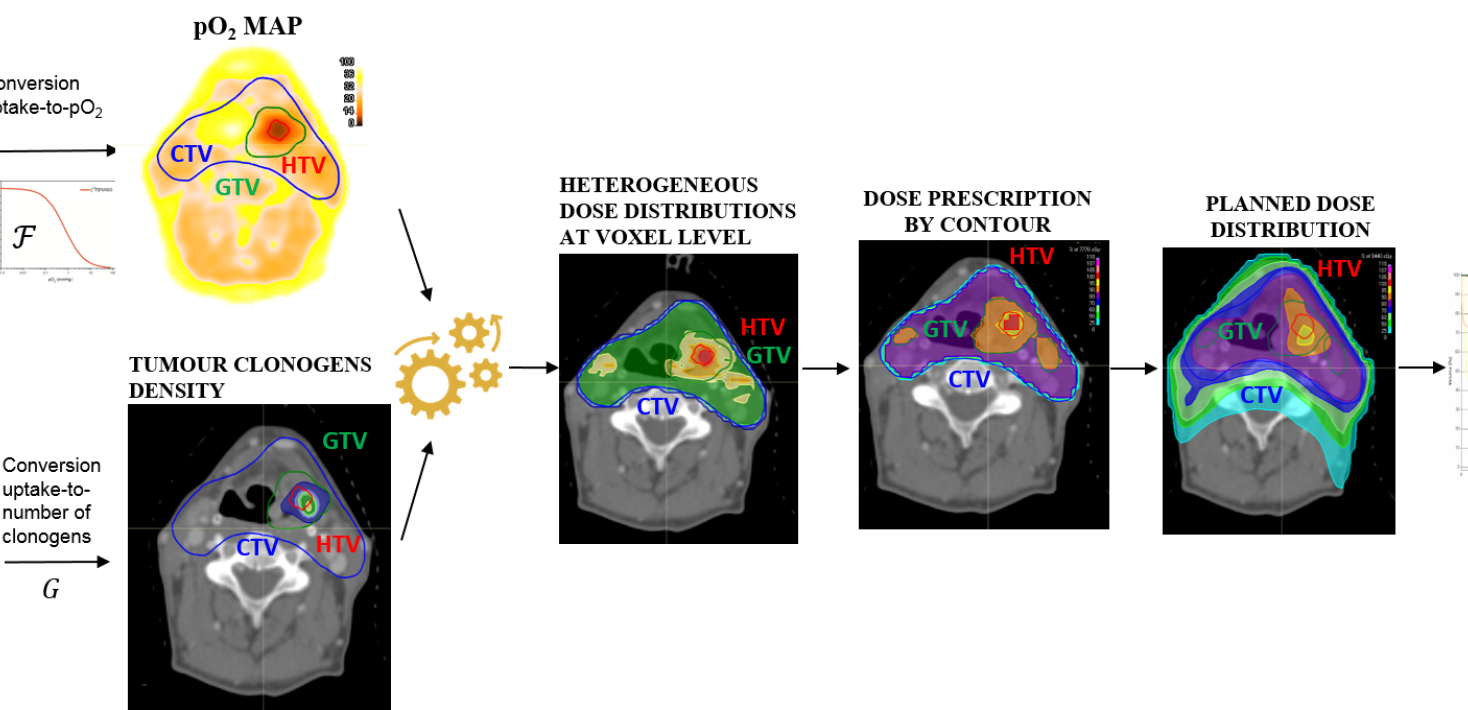
Tailored dose prescription based on the combined information of FDG and FMISO PET images
(a) An existing model for calculating the dose prescription based on tumour hypoxia as derived from FMISO PET images will be further developed to make use of the combined information of FDG and FMISO PET images. This will allow obtaining a personalized dose prescription to achieve a desired level of TCP accounting for both the actual level of hypoxia and the actual density of clonogenic cells. In its current version, the dose prescription model simply assumes a fixed number of clonogenic cells in the whole tumour. However, the distribution of clonogens in a tumour is likely to be heterogeneous and could be derived from FDG avidity as extracted from PET images. Different linear/non-linear conversion functions of FDG uptake into number of clonogenic cells will be considered. For each of these scenarios, the required radiation dose to counteract the increased tumour cell radioresistance at voxel level will be calculated. In silico tested dose prescription strategies will be applied to clinical data with real patient cases so as to assess whether the proposed novel dose escalation strategy, targeting hypoxia and considering a heterogeneous density of clonogens in the tumour based on functional imaging,
is clinically feasible and could be used for individualised radiation therapy.
(b) Spatial correlation between identified tumour sub-regions in need of dose escalation according to the developed theoretical model and the actual tumour recurrence post-treatment available for the considered patient dataset will be analysed, thus serving also as a validation of the
model.
(c) A method for automated treatment planning following the personalized dose prescription strategy based on functional imaging described above will be developed. Automated planning will make possible to handle a large database of patients by saving time and resources in the clinic. An assessment of the performances of automated treatment planning against manual planning by expert planners under no time pressure will be performed.
Treatment response assessment and treatment adaptation based on the time evolution of the hypoxic compartment
(a) An investigation of the evolution of the HTV on pO2 maps derived from FMISO PET images acquired before and during radiochemotherapy will be conducted so as to uncover correlations between extent and severity of hypoxia and treatment outcome.
(b) Treatment adaptation strategies by dose boosting based on the information extracted from repeated FMISO images during treatment will be developed. The boosting strategy will target regions of persistent hypoxia and will make synergistic use of the information on the residual
distribution of clonogenic cells in the tumour. The latter will be available by applying mathematical models of radiation cell kill on the initial clonogenic cell distribution extracted from an available pre-treatment FDG image.
Assessment of the impact of deformable image registration method in hypoxia-based dose painting
When images taken at different time points are considered, questions arise on the type and quality of the performed image registration. The impact of performing a rigid or deformable image registration between the images on boost volume selection and dose level prescription will be
assessed and the registration method of choice determined.
Project members
Project managers
Marta Lazzeroni
Universitetslektor

Members
Iuliana Livia Dasu
Professor